















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
The Mesmerizing World of Spectra: Unveiling the Secrets behind Atoms and Molecules

Have you ever wondered about the fascinating colors that light up the night sky or the rainbow of hues in a fireworks display? These stunning displays are a result of the interactions between atoms and molecules and the light they emit or absorb. Spectra, the beautiful patterns of colors produced by these interactions, have captivated scientists and enthusiasts for centuries. In this article, we will explore the mesmerizing world of spectra, shedding light on their origins and uncovering the secrets hidden within atoms and molecules.
An to Spectra
Spectra, also known as spectral lines, refer to the unique patterns of colored lines or bands that appear when light interacts with matter. These patterns provide crucial information about the atomic and molecular structures of substances and help scientists study their properties in depth.
It all begins with light. When light passes through or interacts with matter, the atoms or molecules in that substance can absorb, emit, or scatter specific wavelengths of light. These interactions produce characteristic colors that form the basis of spectra.
4.6 out of 5
| Language | : | English |
| File size | : | 31529 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| X-Ray for textbooks | : | Enabled |
| Print length | : | 541 pages |
| Lending | : | Enabled |
The Nature of Light
To understand spectra, we must first understand the nature of light. Light, often considered a wave-particle duality, consists of tiny packets of energy called photons. These photons have different wavelengths and frequencies, which determine the properties of the light.
The electromagnetic spectrum encompasses all the possible wavelengths of light, ranging from gamma rays with the shortest wavelengths to radio waves with the longest wavelengths. The visible light spectrum, comprising the colors of the rainbow, falls within a small portion of this larger electromagnetic spectrum.
When light interacts with matter, several phenomena can occur, including reflection, transmission, absorption, and emission. In the case of spectra, we focus primarily on absorption and emission processes.
Atomic Spectra: A Window into the Atomic World
The study of atomic spectra has been pivotal in unraveling the mysteries of the atomic world. Each element in the periodic table has a unique set of energy levels and electron configurations, leading to distinct atomic spectra.
When atoms absorb light, they absorb specific wavelengths corresponding to the energy needed to promote electrons from lower to higher energy levels. This results in dark lines, known as absorption lines, in the overall spectrum.
In contrast, when excited electrons in atoms return to lower energy levels, they emit light at specific wavelengths. These emitted wavelengths produce bright lines, known as emission lines, in the spectrum.
By carefully analyzing these absorption and emission lines, scientists can determine the atomic composition of a substance, its electron configuration, and even its temperature.
Molecular Spectra: Exploring the Building Blocks of Matter
Molecules, consisting of two or more atoms bonded together, possess their own set of unique spectral patterns. Molecular spectra provide invaluable insights into the structure, composition, and behavior of complex substances.
As with atomic spectra, molecular spectra arise from the absorption and emission of light. However, in the case of molecules, the interactions involve not only the electrons but also the rotational and vibrational motion of the atoms within the molecule.
There are three main types of molecular spectra: electronic, vibrational, and rotational spectra. Each spectrum provides different information about the molecule's behavior and properties.
The electronic spectrum reveals details about the transitions of electrons between different energy levels, similar to atomic spectra. It helps scientists identify the electronic configuration of the molecule and study its electronic properties.
Vibrational spectra, on the other hand, focus on the vibrational motion of atoms within a molecule. By studying these spectra, scientists can determine the strength of chemical bonds, identify functional groups, and even distinguish between different isotopes of the same molecule.
Lastly, rotational spectra shed light on the rotational motion of molecules. They provide crucial information about molecular dynamics, such as the size, shape, and symmetry of molecules.
Applications of Spectra
Spectra have broad applications in various scientific disciplines and technological advancements. Their significance spans from astrophysics to chemistry and from medicine to environmental science.
Astrophysicists extensively use spectra to infer the composition and temperature of celestial objects, such as stars and galaxies. By analyzing the light emitted or absorbed by these objects, scientists can determine their chemical makeup and physical properties.
In chemistry, spectra are indispensable tools for identifying unknown substances, studying reaction mechanisms, and characterizing compounds. Spectroscopy techniques are widely used in laboratories and industries to analyze the chemical composition of materials rapidly and accurately.
In medicine, spectra play a vital role in diagnostic techniques such as magnetic resonance imaging (MRI) and infrared spectroscopy. These techniques rely on the unique spectral patterns of molecules to provide invaluable information about the human body, aiding in disease diagnosis and treatment.
Environmental scientists employ spectral data to monitor air quality, detect pollutants, and study climate change. By analyzing the absorption and emission spectra of atmospheric components, researchers can identify the presence and concentration of harmful substances, contributing to environmental preservation efforts.
The Future of Spectra
As technology advances, so does our ability to explore and analyze spectra. With innovative spectroscopic techniques and powerful computational tools, scientists continue to push the boundaries of our understanding of atoms, molecules, and the universe.
Advancements in high-resolution spectroscopy allow for more precise measurements and detailed analysis. Scientists can now obtain spectra with greater accuracy, revealing even finer details about the atomic and molecular world.
In combination with other analytical techniques, spectroscopy facilitates interdisciplinary research, accelerating discoveries in fields such as materials science, nanotechnology, and biophysics. The insights gained from spectra serve as stepping stones towards groundbreaking technologies and scientific breakthroughs.
Unlocking the Beauty of the Spectrum
The study of spectra unveils the enchanting interaction between light and matter, unraveling the secrets hidden within atoms and molecules. Their colors and patterns offer glimpses into the complex world of the microscopic, providing a key to understanding our universe.
As scientists and enthusiasts delve deeper into the realm of spectra, we come one step closer to comprehending the intricacies of nature and harnessing their potential for innovating technologies. So, the next time you gaze at the vibrant hues of a sunset or lose yourself in a mesmerizing fireworks display, remember that behind the captivating beauty lies the captivating science of spectra.
4.6 out of 5
| Language | : | English |
| File size | : | 31529 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| X-Ray for textbooks | : | Enabled |
| Print length | : | 541 pages |
| Lending | : | Enabled |
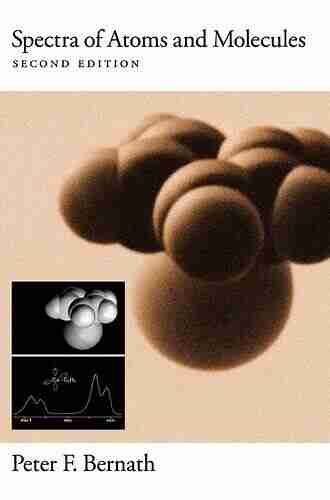
This fourth edition of Peter Bernath's successful Spectra of Atoms and Molecules is designed to provide advanced undergraduate and graduate students a working knowledge of the vast field of spectroscopy. Also of interest to chemists, physicists, astronomers, atmospheric scientists, and engineers, this volume emphasizes the fundamental principles of spectroscopy with the primary goal of teaching the interpretation of spectra. Features include a presentation of group theory as needed to understand spectroscopy, detailed worked examples and a large number of excellent problems at the end of each chapter.
Bernath provides a large number of diagrams and spectra which have been specifically recorded for this book. Molecular symmetry, matrix representation of groups, quantum mechanics, and group theory are among the topics covered; atomic, rotational, vibrational, electronic and Raman spectra are analyzed as well. Bernath's treatment clears the confusing topic of line strengths as needed for quantitative applications. Responding to student requests, the fourth addition features detailed and worked examples in each chapter. This book has also been updated to include the 2018 CODATA revision of physical constants and a large number of corrections and clarifications. New chapters on atmospheric and astronomical spectroscopy have been added. Spectra of Atoms and Molecules demystifies spectroscopy by showing readers the intermediate steps in a derivation, as well as the final result.

 Anthony Burgess
Anthony BurgessEverything You Need To Know About Building Referral...
Are you looking for ways to boost revenue...

 Aleksandr Pushkin
Aleksandr PushkinThe Fascinating History of Afro Uruguay - Unveiling the...
Afro Uruguay refers to the rich and diverse...

 Anton Foster
Anton FosterReflections From Stubborn Son: A Journey of...
Have you ever encountered a stubborn...

 Brennan Blair
Brennan BlairDiscover the Revolutionary World of Protein Modelling:...
Protein modelling is an essential...

 Ricky Bell
Ricky BellThe Best Old Fashioned Advice: Timeless Wisdom Passed...
Have you ever turned to your grandparents,...

 Isaiah Price
Isaiah PriceEmbark on an Unforgettable Journey: The Sword and Sorcery...
Are you ready to be...

 Hassan Cox
Hassan CoxThe Enchanting World of Wendy Darling Comes Alive in...
Step into the magical world of Neverland...

 Ivan Turner
Ivan TurnerAdsorption Calculations And Modelling Chi Tien: Unlocking...
In the field of chemistry, adsorption is a...

 Harvey Hughes
Harvey HughesUnleashing the Full Potential of a Team: How To Organize...
"Genius is 1% inspiration and 99%...

 Desmond Foster
Desmond FosterThe Fascinating Journey of George Romanes: From...
George John Romanes, born on May 20, 1848,...

 Adrien Blair
Adrien BlairThe Untold Truth: The Bible In The Early Church - A...
Lorem ipsum dolor sit amet, consectetur...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Raymond ChandlerUnveiling the Mysteries: The Photographic Story of the Ruined Khmer Temples...
Raymond ChandlerUnveiling the Mysteries: The Photographic Story of the Ruined Khmer Temples...
 Junot DíazUnveiling the Intriguing Tapestry of Popular Culture Between Two Revolutions:...
Junot DíazUnveiling the Intriguing Tapestry of Popular Culture Between Two Revolutions:...
 Luke BlairMalaysia Missing Plane: The Full Story - Shocking Revelations and Unanswered...
Luke BlairMalaysia Missing Plane: The Full Story - Shocking Revelations and Unanswered... Wade CoxFollow ·11.8k
Wade CoxFollow ·11.8k Randy HayesFollow ·17.2k
Randy HayesFollow ·17.2k Harvey HughesFollow ·4.8k
Harvey HughesFollow ·4.8k Italo CalvinoFollow ·17k
Italo CalvinoFollow ·17k John GreenFollow ·6.8k
John GreenFollow ·6.8k Camden MitchellFollow ·19.3k
Camden MitchellFollow ·19.3k Bradley DixonFollow ·2.9k
Bradley DixonFollow ·2.9k Isaiah PowellFollow ·13.5k
Isaiah PowellFollow ·13.5k