















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
The Fascinating World of Chemical Equilibria, Chemical Engineering, and Thermodynamics!

Chemical engineering is a captivating field that combines principles of chemistry and engineering to solve complex problems and improve industrial processes. One of the fundamental concepts in chemical engineering is chemical equilibrium, which plays a crucial role in designing efficient systems and understanding the behavior of chemical reactions. In this article, we will delve into the depths of chemical equilibria and explore the fascinating world of chemical thermodynamics.
Understanding Chemical Equilibrium
In chemical engineering, equilibrium refers to a state where the forward and reverse reactions of a chemical reaction occur at equal rates, resulting in no net change in the concentrations of reactants and products. This concept can be visualized as a balancing act where the reaction tries to reach a state of balance.
To understand chemical equilibrium, let's consider the example of the Haber process, which is used to produce ammonia. The reaction can be represented as:
4.4 out of 5
| Language | : | English |
| File size | : | 3353 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Word Wise | : | Enabled |
| Print length | : | 211 pages |
| Lending | : | Enabled |
N2(g) + 3H2(g) ⇌ 2NH3(g)
Initially, as the reactants N2 and H2 are introduced into the system, the forward reaction proceeds rapidly and forms ammonia. However, as the concentration of NH3 increases, the reverse reaction also gains momentum. Eventually, the rates of the forward and reverse reactions become equal, achieving a state of equilibrium.
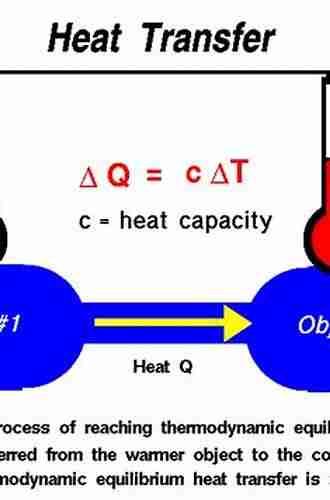
Chemical equilibrium is governed by the principles of thermodynamics, which allow us to predict and understand the behavior of chemical reactions. Thermodynamics provides the tools to quantify properties such as enthalpy, entropy, and free energy, which are essential in determining the feasibility of a reaction.
Chemical Thermodynamics
Chemical thermodynamics is the branch of thermodynamics that deals specifically with chemical reactions and their energetics. It enables engineers to assess the efficiency and feasibility of chemical processes by considering factors such as energy transfer, heat exchange, and spontaneity of reactions.
One of the key concepts in chemical thermodynamics is the Gibbs free energy, denoted as ΔG. It provides a measure of the energy available to do useful work during a chemical reaction. The sign and magnitude of ΔG can determine whether a reaction is exergonic (releasing energy) or endergonic (consuming energy).
For a reaction to occur spontaneously, the change in Gibbs free energy must be negative, indicating an exergonic reaction. However, when ΔG is positive, the reaction requires an input of energy to proceed, making it endergonic. By analyzing the values of ΔG, engineers can optimize reaction conditions and design processes that are thermodynamically favorable.
Applications in Chemical Engineering
Chemical equilibria and thermodynamics find extensive applications in chemical engineering across various industries. Some of these applications include:
Reaction Kinetics:
The study of chemical equilibria helps engineers determine the optimal conditions for reactions, such as temperature, pressure, and catalysts. This knowledge enables the design of efficient reactors and maximizes product yields.
Process Design and Optimization:
Thermodynamics plays a vital role in process design and optimization. Engineers can predict the behavior of chemical reactions and optimize operating conditions to minimize energy consumption, reduce waste generation, and increase production efficiency.
Chemical Separation:
Understanding the principles of chemical equilibrium allows engineers to design separation processes such as distillation, extraction, and chromatography. These processes are crucial for isolating desired products and purifying chemical compounds.
Phase Equilibria:
Chemical engineers utilize thermodynamics to study the behavior of mixtures in different phases. This knowledge is particularly relevant in designing separation processes, as well as in the formulation and optimization of materials such as polymers and pharmaceuticals.
The Future of Chemical Engineering
As the world continues to face challenges related to sustainability and energy consumption, chemical engineering, alongside thermodynamics and chemical equilibria, will play a crucial role in developing innovative solutions.
Advancements in areas such as renewable energy, green chemistry, and sustainable manufacturing will rely heavily on the principles of chemical engineering. The understanding and application of chemical equilibria and thermodynamics will enable engineers to design cleaner and more efficient processes that minimize environmental impact and maximize resource utilization.
Chemical equilibria, chemical engineering, and thermodynamics converge to form a captivating and essential field of study. The principles and concepts discussed in this article provide a glimpse into the intricacies of chemical reactions and their energetics.
Chemical engineers utilize their knowledge of chemical equilibria and thermodynamics to design efficient processes, optimize reaction conditions, and develop sustainable technologies. The applications are vast, from developing more effective medications to designing greener manufacturing processes.
So, whether you aspire to pursue a career in chemical engineering or simply wish to unravel the secrets behind the chemical reactions that shape our world, understanding chemical equilibria and the principles of thermodynamics will undoubtedly make your journey even more enriching and exciting!
4.4 out of 5
| Language | : | English |
| File size | : | 3353 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Word Wise | : | Enabled |
| Print length | : | 211 pages |
| Lending | : | Enabled |
The book offers advanced students, in 7 volumes, successively characterization tools phases, the study of all types of phase, liquid, gas and solid, pure or multi-component, process engineering, chemical and electrochemical equilibria, the properties of surfaces and phases of small sizes. Macroscopic and microscopic models are in turn covered with a constant correlation between the two scales. Particular attention was given to the rigor of mathematical developments.
Besides some very specialized books, the vast majority of existing works are intended for beginners and therefore limited in scope. There is no obvious connection between the two categories of books, general books does not go far enough in generalizing concepts to enable easy reading of advanced literature. The proposed project aims to give readers the ability to read highly specialized publications based on a more general presentation of the different fields of chemical thermodynamics. Consistency is ensured between the basic concepts and applications. So we find, in the same work, the tools, their use and comparison, for a more general macroscopic description and a microscopic description of a phase.

 Anthony Burgess
Anthony BurgessEverything You Need To Know About Building Referral...
Are you looking for ways to boost revenue...

 Aleksandr Pushkin
Aleksandr PushkinThe Fascinating History of Afro Uruguay - Unveiling the...
Afro Uruguay refers to the rich and diverse...

 Anton Foster
Anton FosterReflections From Stubborn Son: A Journey of...
Have you ever encountered a stubborn...

 Brennan Blair
Brennan BlairDiscover the Revolutionary World of Protein Modelling:...
Protein modelling is an essential...

 Ricky Bell
Ricky BellThe Best Old Fashioned Advice: Timeless Wisdom Passed...
Have you ever turned to your grandparents,...

 Isaiah Price
Isaiah PriceEmbark on an Unforgettable Journey: The Sword and Sorcery...
Are you ready to be...

 Hassan Cox
Hassan CoxThe Enchanting World of Wendy Darling Comes Alive in...
Step into the magical world of Neverland...

 Ivan Turner
Ivan TurnerAdsorption Calculations And Modelling Chi Tien: Unlocking...
In the field of chemistry, adsorption is a...

 Harvey Hughes
Harvey HughesUnleashing the Full Potential of a Team: How To Organize...
"Genius is 1% inspiration and 99%...

 Desmond Foster
Desmond FosterThe Fascinating Journey of George Romanes: From...
George John Romanes, born on May 20, 1848,...

 Adrien Blair
Adrien BlairThe Untold Truth: The Bible In The Early Church - A...
Lorem ipsum dolor sit amet, consectetur...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Gabriel Garcia MarquezNo Regrets An American Adventure In Afghanistan: A Tale of Bravery and...
Gabriel Garcia MarquezNo Regrets An American Adventure In Afghanistan: A Tale of Bravery and... Jace MitchellFollow ·15.2k
Jace MitchellFollow ·15.2k Henry JamesFollow ·5.8k
Henry JamesFollow ·5.8k William FaulknerFollow ·11k
William FaulknerFollow ·11k Brayden ReedFollow ·9.9k
Brayden ReedFollow ·9.9k Harvey BellFollow ·15.7k
Harvey BellFollow ·15.7k Kevin TurnerFollow ·9.8k
Kevin TurnerFollow ·9.8k Devon MitchellFollow ·9.1k
Devon MitchellFollow ·9.1k Sidney CoxFollow ·17.4k
Sidney CoxFollow ·17.4k